abbott point of care covid test
COVID-19 Antibody Test Texas Abbott COVID-19 Antibody Test Locations In the event of a medical emergency dial 911 or visit your closest emergency room immediately. Currently the Virginia Department of Health VDH offers one type of prescription antigen test.

Abbott Id Now Covid 19 Detection Test System Us
The COVID-19 pandemic is affecting all of us around the world.
. Reporting Requirements for Rapid Testing in Point-of-Care Settings. Abbott ID NOW POC PCR testing creates faster access to COVID-19 testing and immediate access to test results for health-care workers and their families. This study recruited participants presenting for COVID-19 testing at three Melbourne metropolitan hospitals during a period of low COVID-19 prevalence.
Nursing homes and assisted living facilities may use rapid antigen point-of-care POC tests to test personnel residents or visitors for COVID-19. The Abbott PanBio TM COVID-19 Ag point-of-care test was performed alongside RT-PCR. The Food and Drug Administration FDA has issued an Emergency Use Authorization for the Abbott ID Now COVID-19 test a molecular point-of-care test that delivers results within minutes allowing healthcare professionals to make clinical decisions during a patient visit.
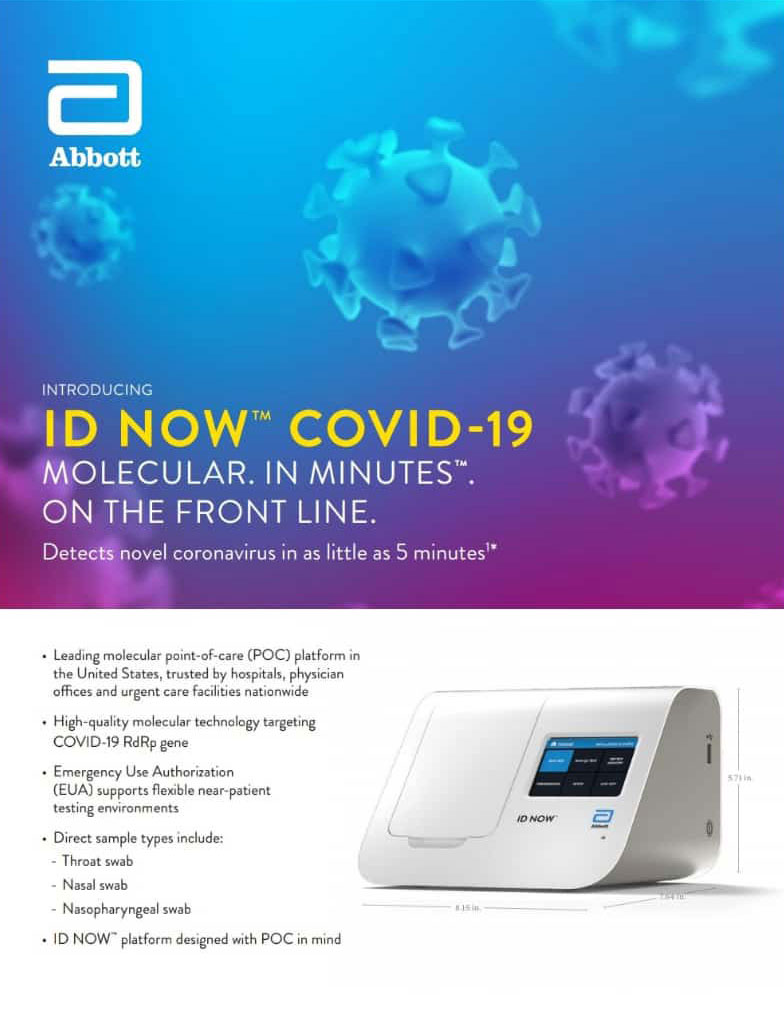
The ID NOW turnaround time for positive results can be as little as 5 minutes with negative results in 13 minutes. Food and Drug Administration FDA for the fastest available molecular point-of-care test for the detection of novel coronavirus COVID-19 delivering positive results in as little as five minutes and negative results in 13 minutes. The new access to POC PCR testing through the HCW Front of the Line Program will provide teams with additional capacity to support the fight against COVID-19.
Personnel and residents who have symptoms of COVID-19 regardless of their vaccination status. T his test is authorized for use at the Point of Care POC ie in patient care settings operating under a CLIA Certificate of Waiver Certificate of Compliance or Certificate of Accreditation. Abbott has received emergency use authorization EUA from the US.
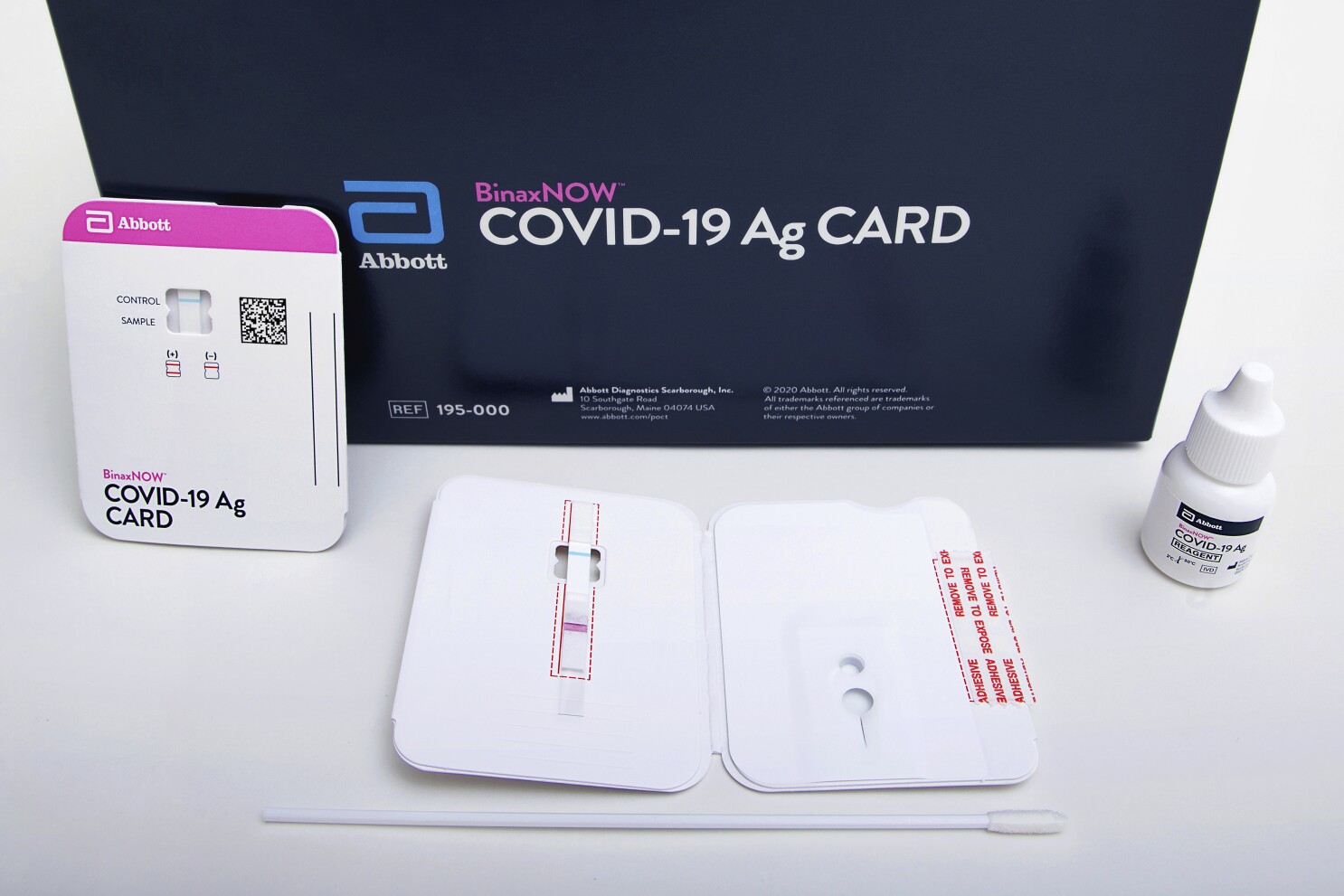
Current performance of COVID-19 test methods and devices and proposed performance criteria Working document of Commission services. The BinaxNOW COVID-19 Antigen Self Test is a lateral flow immunoassay intended for the qualitative detection of nucleocapsid protein antigen from SARS-CoV-2 from individuals with or without symptoms or other epidemiological reasons to suspect COVID-19 infection when tested twice over three days with at least 36 hours between tests. The Food and Drug Administration FDA has issued an Emergency Use Authorization for the Abbott ID Now COVID-19 test a molecular point-of-care test that delivers results within minutes allowing healthcare professionals to make clinical decisions during a patient visit.
Submit by fax to the Washington State Department of Health at 206 512-2126. Expiration Dates of SELECT Lots of the Point-of-Care Abbott BinaxNow COVID-19 Antigen Tests EXTENDED to 15 months. Abbotts rapid COVID-19 test isnt the only point-of-care test to receive FDA authorization during the pandemic but Trump has touted it the most by far hailing the speed at which results can.
CLIA-certified laboratories or testing sites are no longer required to report negative results for non-NAAT. COVID-19 TESTS WHEN YOU NEED THEM Abbott is creating tests to help detect the SARS-CoV-2 virus and better understand the spread of COVID-19 REFERENCES. According to Abbott the rapid test which runs on the ID NOW platform is an.
Abbott ID NOW COVID -19 BD Veritor System for Rapid Detection of SARS -CoV-2 Cepheid Xpert Xpress SARS-CoV-2 test. As a leader in diagnostic testing we have a unique responsibility to contribute our expertise to help fight the COVID-19 pandemic. Abbott BinaxNOW COVID-19 Antigen Ag Card A self-contained antigen test that uses a card and does not require a separate analyzer device.
Abbott is putting its resources towards helping you navigate this crisis. ABBOTT ID Now COVID-19 POINT OF CARE TESTING 5192020 The following guidance is for institutions that have an Abbott ID NOW instrument and test kits for performing CLIA-waived rapid point of care COVID-19 testing. To help provide the critical diagnostic information needed Abbott is currently providing and.
Allocation and distribution of instruments and test kits will be determined by Central. WASHINGTON STATE COVID-19 POINT OF CARE TEST RESULT REPORT FORM Complete one form per result. It is important to evaluate the performance of point-of-care nucleic acid amplification and antigen assays to implement workflows such as reflexing negative samples to overcome assay performance limitations in sensitivity and risk of.
Please see the following link to determine if you currently. This document provides a step-by-guide to get started with Point of Care POC COVID-19 antigen testing. What makes this test so different is where it can be used.
The expiration dates of the lot numbers listed in the referenced link have been extended to 15 months from the date of manufacture. A CLIA-certified laboratory or testing site must report all positive SARS-CoV-2 diagnostic and screening test results to the person who was tested or that persons healthcare provider. European Commission April 16 2020.
Rapid COVID testing in settings such as the emergency department could improve time to diagnosis and expedite patient management. The BinaxNOW COVID-19 Antigen Self Test is a lateral flow immunoassay intended for the qualitative detection of nucleocapsid protein antigen from SARS-CoV-2 from individuals with or without symptoms or other epidemiological reasons to suspect COVID-19 infection when tested twice over three days with at least 36 hours between tests. Abbotts molecular point-of-care test for COVID-19 delivers positive results in as little as five minues and negative results in 13 minutes.
Who can use Abbott ID NOW for point of care PCR. In addition participants with COVID-19 notified to the Victorian Government were invited to provide additional swabs to. The Rhode Island Department of Health RIDOH strongly encourages facilities to test.
According to Abbott the rapid test which runs on the ID NOW platform is an. The ID NOW COVID-19 assay Abbott USA is a rapid instrument-based molecular isothermal amplification test for the detection of SARS-CoV-2 from oropharyngeal nasal and nasopharyngeal swabs NPS. Personnel and residents who.

Steps To Use Id Now Effectively Abbott Newsroom

Id Now Covid 19 Abbott Point Of Care

Panbio Covid 19 Ag Rapid Test Device Abbott Point Of Care

Indonesia Go Id Cartridge Nya Isi Ulang Diagnosisnya Lima Menit

14 000 Rapid Covid 19 Testing Kits Coming To Grey Bruce Ctv News

As Problems Grow With Abbott S Fast Covid Test Fda Standards Are Under Fire Kaiser Health News

Fda Authorizes Covid 19 Test That Doesn T Need Special Equipment Los Angeles Times

Point Of Care Testing Diagnostics Testing Newsroom
Abbott S Point Of Care Covid 19 Test Detects Coronavirus In As Little As 5 Minutes Biospace

Laboratory Id Now Abbott Point Of Care Test Pcr Mobile Device Autodoc

Laboratory Id Now Abbott Point Of Care Test Pcr Mobile Device Autodoc
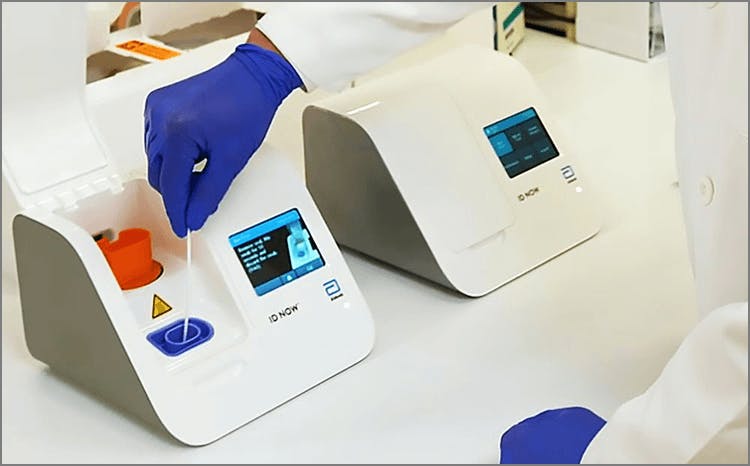
Covid 19 Rapid Molecular Swab Test Good Doctors Medical Centre
Laboratory Id Now Abbott Point Of Care Test Pcr Mobile Device Autodoc
/cdn.vox-cdn.com/uploads/chorus_asset/file/19856029/IDNOW_INACTION3_macro_300dpi_1200x628.jpg)
A New Covid 19 Test Can Return Results In 5 Minutes The Verge
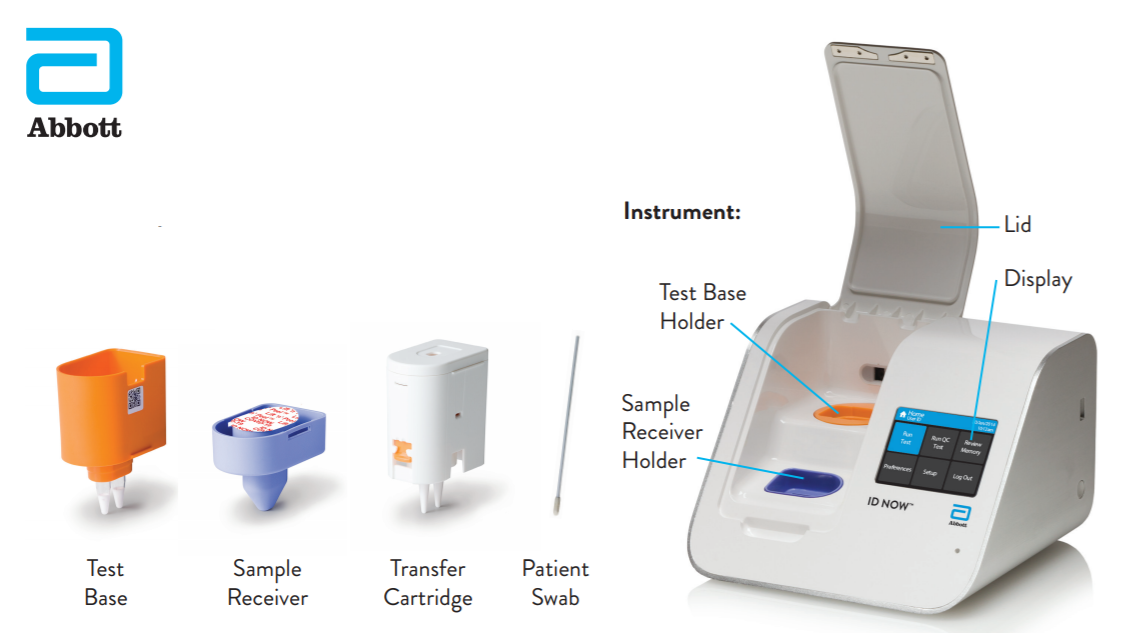
Instant Results From Abbotts Covid 19
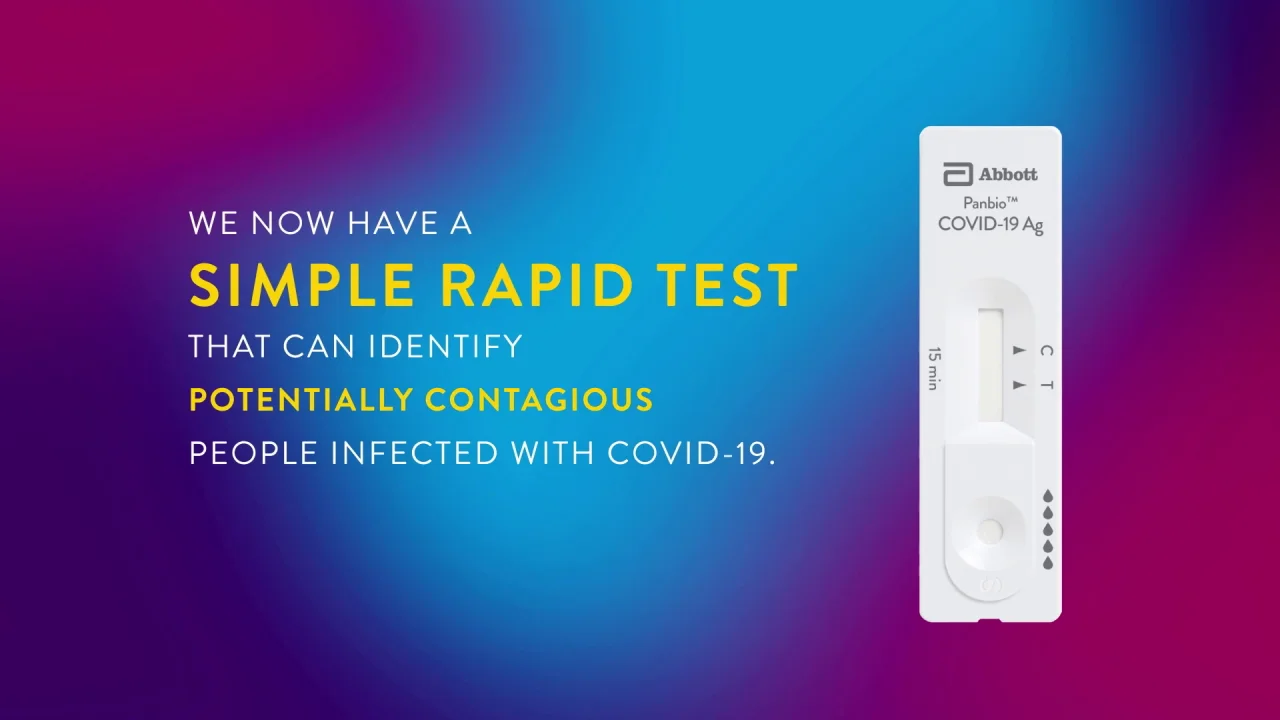
Panbio Covid 19 Ag Test Abbott Point Of Care

Panbio Covid 19 Igg Igm Rapid Test Abbott Point Of Care

Abbott Id Now Covid 19 Instructions Modified

Abbott Labs Rapid 5 Covid 19 Test To Fill In Testing Gaps For Millions In The U S